Gram Staining
The purpose of a gram stain is to identify the bacteria by dividing them into either gram-positive or gram-negative groups. And as always, we did this to observe the cell shape, arrangement, and size of the bacteria.
The materials we used were:
slide with fixed smear of bacteria
crystal violet stain
Gram's iodine
95% ethanol
safranin
staining rack of sink
wash bottle with deionized water
biblulous paper
forceps
compound microscope
immersion oil
 |
| Fixing the smear |
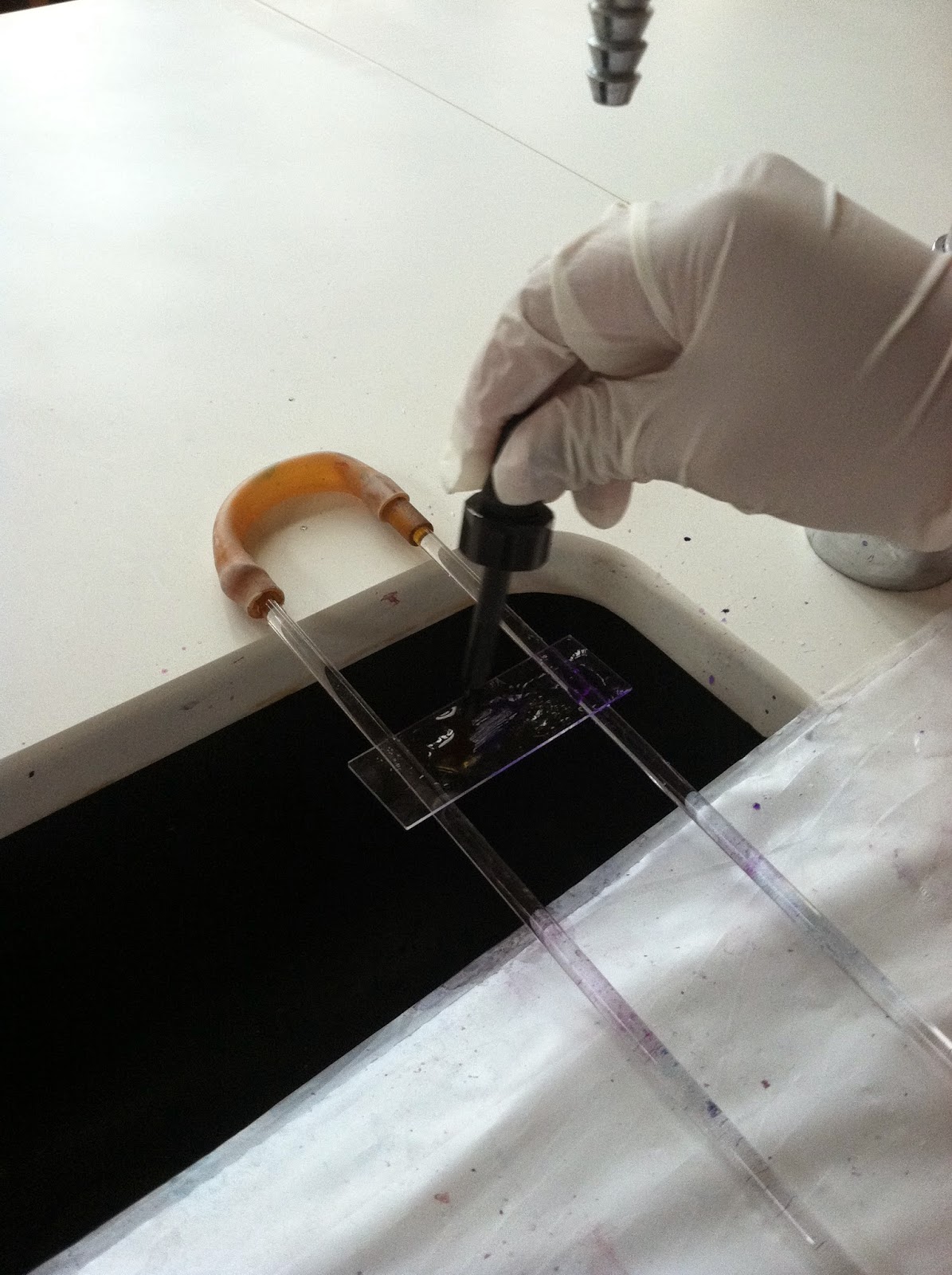
First, we placed the slide on the staining rack so it wouldn't fall into the abyss of the sink. Then, after covering the bacteria with crystal violet stain for 20 seconds, we rinsed the slide with water to remove the excess stain.
Next, we covered it with Gram's iodine for 1 minute and rinsed that off too. Our bacteria just isn't ready for that kind of commitment. Then we decolorized the bacteria with 95% ethanol by holding the slide at a 45 degree angle. we added the decolorizing agent drop by drop until the color stopped running. Again, we immediately rinsed off the slide to remove the decolorizing agent.
We then covered the smear with safranin for one minute and then proceeded to rinse the excess off.
After blotting with bibulous paper and marking the area with a China marker, we observed it under the microscope with the immersion oil.
After observation, our bacteria showed results of being gram-negative. We described it as reddish in color and consisting of small rounded rods.
Capsule Staining:
The purpose
of this staining technique was to view the bacterial capsules or slime layers
of the unknown bacteria. In this experiment, we had to prepare a smear of
bacteria in nigrosin to use for the capsule staining, so we'll mention how to
do that first.
This method
required our unknown bacteria (from the petri plate to be specific), nigrosin
stain (the black stuff), 2 microscope slides (clean please), and an inoculating
loop (sterilized).
In order to prepare the bacteria smear in nigrosin, we first placed a drop of the nigrosin on one end of the microscope slide. Following the aseptic technique, we transferred a small amount of bacteria from the petri plate into the nigrosin drop. We mixed it as well as we could within such a small parameter. Next, we took the second microscope slide and held it at a 45 degree angle in the bacteria-nigrosin drop. But we didn't stop there. we used the second slide to smear the drop along the first microscope slide until the smear was a thin film with a feathered edge at the trailing end. Then, we waited for the smear to completely air dry.
As for the real deal (the capsule
staining), we needed a few more materials to carry out this process.
More
Materials:
safranin
staining
rack over a sink
wash bottle
with deionized water
compound
microscope
immersion
oil
After the smear air dried, we covered it with the safranin and tender
loving care. Then, we gently rinsed off any excess stain with the water, making
sure not to over rinse.
We blotted the slide with the bibulous paper and examined the smear under the microscope
Endospore Staining
The purpose
of this stain was to view the bacterial endospores under the
microscope and to observe the location of an endospore in a sporulating
cell. Endospores are formed by a few types of gram-positive bacteria. The
purpose of endospores is to ensure the survival of a particular kind of
bacteria. They are specialized for survival in harsh conditions. Equipped with
a thick protein coat, endospores are resistant to most staining. However, the
use of heat helped to permeate the malachite green stain into the spore coat.
The
materials used were:
slide with
fixed smear of bacteria
malachite
green stain
safranin
staining rack
steaming
water in a large beaker
hot plate
for heating the water
piece of
filter paper
wash bottle
with deionized water
bibulous paper
forceps
microscope
immersion
oil
We stained the bacteria for 5-6 minutes and added additional stain as it evaporated. Afterwards, we used the forceps to remove the filter paper from the slide and placed it in a biohazard bag (proper disposal is no joke, kids).
After allowing the slide to cool, we
rinsed the slide with water for about 30 seconds to removed the excess
malachite green stain. We then covered the smear with safranin for about 75
seconds and rinsed with with water yet again to remove the excess stain. We
blotted the water from the slide with the bibulous paper before viewing it
under the microscope with immersion oil.
We observed that our bacteria had very small, round spores within the bacteria.
Acid-Fast Staining
The purpose
of this last and final stain of the day was to distinguish bacteria based on
the lipid content of their cell walls: acid-fast and non-acid-fast. We did not
get to use our beloved "N" unknown bacteria, but instead Dr. P
provided us with the bacteria that we were to use.
This
experiment required:
microscope
slide with fixed smear of bacteria
Ziehl-Neelsen
carbolfuchsin
acid-alcohol
methylene
blue
hot plate
large beaker
filled with water
wash bottle
with deionized water
filter paper
staining
rack
bibulous
paper
forceps
compound
microscope
immersion
oil
perseverance
After boiling the water on the hot plate, we placed the staining rack on the beaker and the bacteria smear on top of that. We placed the filter paper on top of the slide and saturated it with the Zeihl-Neelsen carbolfuchsin, making sure it didn't dry out. We stained the bacteria for 3-5 minutes. Next, we used the forceps to remove the paper from the slide and, once again, disposed of it properly in a biohazard bag. We allowed the slide to cool before going forth with other shenanigans.
After
cooling, we placed the staining rack over the sink and rinsed the slide with
water to remove any excess stain. We then used acid-alcohol to decolorize the
bacteria by holding it at a 45 degree angle and adding drop by drop until the
color stopped running. We immediately rinsed the slide to remove the
deolorizing agent. We covered the smear with methylene blue for 2 minutes but
inevitably rinsed that off as well. After blotting the slide with bibulous
paper, we examined the smear under the microscope with immersion oil.









































No comments:
Post a Comment